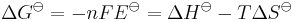Reactivity series
In introductory chemistry, the reactivity series or activity series is an empirical series of metals, in order of "reactivity" from highest to lowest.[1][2][3] It is used to summarize information about the reactions of metals with acids and water, single displacement reactions and the extraction of metals from their ores.
-
Metal Ion Reactivity Extraction K K+ reacts with water electrolysis Na Na+ Li Li+ Rb Rb+ Ba Ba2+ Sr Sr2+ Ca Ca2+ Mg Mg2+ reacts with acids Al Al3+ C included for comparison Mn Mn2+ reacts with acids smelting
with cokeZn Zn2+ Cr Cr2+ Fe Fe2+ Cd Cd2+ Co Co2+ Ni Ni2+ Sn Sn2+ Pb Pb2+ H2 H+ included for comparison Sb Sb3+ may react with some strongly oxidizing acids heat or
physical
extractionBi Bi3+ Cu Cu2+ Ag Ag+ Hg Hg2+ Au Au3+ Pt Pt2+
Going from bottom to top the metals:
- increase in reactivity;
- lose electrons more readily to form positive ions;
- corrode or tarnish more readily;
- require more energy (and different methods) to be separated from their ores;
- become stronger reducing agents.
Contents |
Defining reactions
There is no unique and fully consistent way to define the reactivity series, but it is common to use the three types of reaction listed below, many of which can be performed in a high-school laboratory (at least as demonstrations).
Reaction with water and acids
The most reactive metals, such as sodium, will react with cold water to produce hydrogen and the metal hydroxide:
- 2 Na (s) + 2 H2O (l) → 2 NaOH (aq) + H2 (g)
Metals in the middle of the reactivity series, such as iron, will react with acids such as sulphuric acid (but not water at normal temperatures) to give hydrogen and a metal salt, such as iron(II) sulphate:
- Fe (s) + H2SO4 (aq) → FeSO4 (aq) + H2 (g)
There is some ambiguity at the borderlines between the groups. Magnesium, aluminium and zinc can react with water, but the reaction is usually very slow unless the metal samples are specially prepared to remove the surface layer of oxide which protects the rest of the metal. Copper and silver will react with nitric acid; but because nitric acid is an oxidizing acid, the oxidizing agent is not the H+ ion in normal acids, but the NO3− ion.
Single displacement reactions
An iron nail placed in a solution of copper sulfate will quickly change colour as metallic copper is deposited and the iron is converted into iron(II) sulphate:
- Fe (s) + CuSO4 (aq) → Cu (s) + FeSO4 (aq)
In general, a metal can displace any of the metals which are lower in the reactivity series: the higher metal reduces the ions of the lower metal. This is used in the thermite reaction for preparing small quantities of metallic iron, and in the Kroll process for preparing titanium (Ti comes at about the same level as Al in the reactivity series). For example, aluminium will reduce Iron(III) oxide to iron, becoming aluminium oxide in the process:
- Al (s) + Fe2O3 (s) → Fe (s) + Al2O3 (s)
Similarly, magnesium can be used to extract titanium from titanium tetrachloride, forming magnesium chloride in the process:
- 2 Mg (s) + TiCl4 (l) → Ti (s) + 2 MgCl2 (s)
However, other factors can come into play, such as in the preparation of metallic potassium by the reduction of potassium chloride with sodium at 850 °C. Although sodium is lower than potassium in the reactivity series, the reaction can proceed because potassium is more volatile, and is distilled off from the mixture.
- Na (g) + KCl (l) → K (g) + NaCl (l)
Comparison with standard electrode potentials
The reactivity series is sometimes quoted in the strict reverse order of standard electrode potentials, when it is also known as the "electrochemical series":
- Li > K > Sr > Ca > Na > Mg > Al > Zn > Cr > Fe > Cd > Co > Ni > Sn > Pb > H > Cu > Ag > Hg > Pt > Au
The positions of lithium and sodium are changed on such a series: gold and platinum are also inverted, although this has little practical significance as both metals are highly unreactive.
Standard electrode potentials offer a quantitative measure of the power of a reducing agent, rather than the qualitative considerations of other reactivity series. However, they are only valid for standard conditions: in particular, they only apply to reactions in aqueous solution. Even with this proviso, the electrode potentials of lithium and sodium – and hence their positions in the electrochemical series – appear anomalous. The order of reactivity, as shown by the vigour of the reaction with water or the speed at which the metal surface tarnishes in air, appears to be
- potassium > sodium > lithium > alkaline earth metals,
the same as the reverse order of the (gas-phase) ionization energies. This is borne out by the extraction of metallic lithium by the electrolysis of a eutectic mixture of lithium chloride and potassium chloride: lithium metal is formed at the cathode, not potassium.[4]
Anomalous electrode potential of lithium
The standard electrode potential of a reaction Eo is related to the Gibbs free energy change ΔGo and the enthalpy change ΔHo by
| M = | Li | Na | K |
|---|---|---|---|
| ΔatH |
+162 | +110 | +90 |
| Ei / kJ mol−1 | +520 | +496 | +419 |
| ΔhydrH |
–960 | –846 | –761 |
| ΔfH |
–278 | –240 | –252 |
| ΔfS |
+51 | +73 | +104 |
| ΔfG |
–293 | –261 | –283 |
| E |
+3.04 | +2.71 | +2.93 |
| Sources: Jolly (1991),[5] Greenwood & Earnshaw (1984).[4][6] |
|||
The same relation can be applied to the half-reactions of tabulated standard electrode potentials, as the energy associated with the electron(s) will cancel whenever a full reaction is considered and so can be ignored. The enthalpy change of the half-reaction can be further analyzed as a Born–Haber cycle, and shown to be equal to the enthalpy change of atomisation ΔatHo plus the ionization energy Ei plus the enthalpy change of hydration ΔhydrHo of the gaseous ions. There is a further contribution from the entropy change of the half-reaction, ΔSo. Values of these quantities for lithium, sodium and potassium are given in the table (note that the reaction is shown for the formation of M+, which is the reverse of the reduction reaction for which standard electrode potentials are conventionally quoted).
The high electrode potential of the Li/Li+ couple can be seen to be entirely due to the unusually large enthalpy change of hydration of the gaseous Li+ ion, which overrides the other three factors. The lithium ion is small and highly polarising, and so binds the coordinated water molecules very tightly. This can also be seen in the relatively low positive entropy change for the formation of Li+(aq): the presence of lithium ions in solution creates more order in the solvent (water) than for either sodium or potassium ions.
The reaction of metallic lithium with water is more exothermic than any other alkali metal (many times more exothermic for a given mass or volume), but it is less vigorous for kinetic reasons. The enthalpy change of atomisation is twice as high for lithium than for potassium, indicating stronger bonding in the metal (both metals have the same body-centered cubic structure). Another way to measure an element's reactivity relative to other elements, is to look at its electronegativity. This is most useful when looking at how anions might replace other anions in a reaction. Anions with greater electronegativity values are more reactive and will replace anions with lower electronegativity values.
References
- ^ France, Colin (2008), The Reactivity Series of Metals, http://www.gcsescience.com/r1-reactivity-series-metals.htm
- ^ Briggs, J. G. R. (2005), Science in Focus, Chemistry for GCE 'O' Level, Pearson Education, p. 172
- ^ Lim Eng Wah (2005), Pearson Education, p. 190
- ^ a b Greenwood, Norman N.; Earnshaw, A. (1984). Chemistry of the Elements. Oxford: Pergamon. pp. 82–87. ISBN 0-08-022057-6.
- ^ Jolly, William L. (1991), Modern Inorganic Chemistry (2nd ed.), New York: McGraw-Hill, pp. 586–87, ISBN 0-07-032768-8
- ^ The values for the enthalpy changes of hydration of Li+ (−520 kJ/mol) and Na+ (−405 kJ/mol) given in Greenwood & Earnshaw (1984) are inconsistent with the other data quoted in the same source and certainly far too low. The enthalpy changes for the gas-phase reactions Li+(g) + 6H2O → [Li(H2O)6]+ and Na+(g) + 5H2O → [Na(H2O)5]+ have been measured as −529(16) kJ/mol and −347(14) kJ/mol respectively: Rodgers, M. T.; Armentrout, P. B. (2000), "Noncovalent Metal-Ligand Bond Energies as Studied by Threshold Collision-Induced Dissociation", Mass Spectrom. Rev. 19 (4): 215, doi:10.1002/1098-2787(200007)19:4<215::AID-MAS2>3.0.CO;2-X, PMID 10986693. The enthalpy changes of hydration quoted in the table have been calculated from the enthalpy changes of formation of the ions in solution.
- Holt, Reinhart, and Winston: Modern Chemistry. Page 286. Copyright 2006.
See also the Wikipedia entry Reactivity (chemistry), which discusses the inconsistent way that the term 'reactivity' is used in chemistry.